Equilibrium Reactions Acids And Bases Quick Review Notes: Your Ultimate Study Guide

4.3 out of 5
| Language | : | English |
| File size | : | 105 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Print length | : | 6 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
Equilibrium reactions are a fundamental concept in chemistry that plays a crucial role in understanding various chemical processes. Equilibrium reactions involving acids and bases are particularly important in fields such as biochemistry, environmental chemistry, and industrial chemistry.
This comprehensive guide provides a quick review of equilibrium reactions acids and bases, covering key concepts, equations, and problem-solving techniques. It is designed to help students, researchers, and professionals refresh their knowledge and enhance their understanding of this essential topic.
Key Concepts
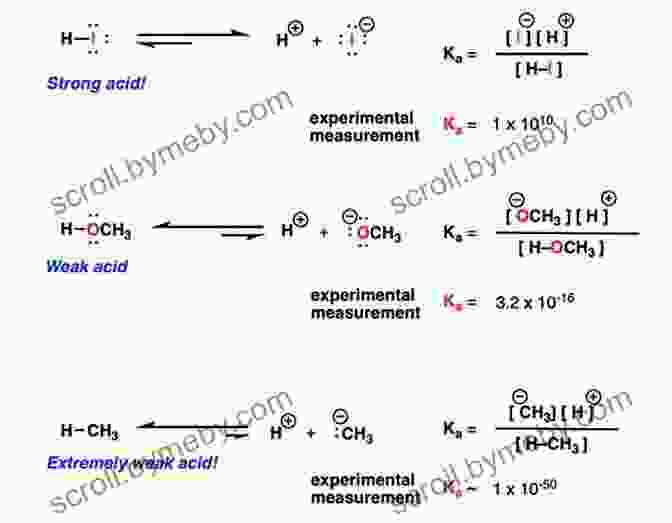
- Acids and Bases: Acids are substances that donate protons (H+),while bases are substances that accept protons.
- pH: pH is a measure of the acidity or basicity of a solution. It is calculated as the negative logarithm of the hydrogen ion concentration [H+].
- pKa and Kb: pKa is the negative logarithm of the acid dissociation constant, while Kb is the negative logarithm of the base dissociation constant. These values indicate the strength of an acid or base.
- Kw: Kw is the ion product constant for water, which is equal to 1.0 x 10^-14 at 25°C.
- ICE Tables: ICE tables (Initial, Change, Equilibrium) are used to track the concentrations of reactants and products at different stages of an equilibrium reaction.
- Le Chatelier's Principle: Le Chatelier's principle predicts the direction in which an equilibrium reaction will shift when a change is made to the system.
Equilibrium Reactions Acids and Bases
Equilibrium reactions involving acids and bases can be represented by the following general equation:
HA + H2O H3O+ + A-
Where HA is the acid, H2O is water, H3O+ is the hydronium ion, and A- is the conjugate base of the acid.
The equilibrium constant (Keq) for this reaction is given by:
Keq = [H3O+][A-] / [HA]
Problem-Solving Techniques
Solving equilibrium reactions acids and bases problems involves applying the following steps:
- Write the balanced chemical equation.
- Create an ICE table.
- Substitute the equilibrium concentrations into the equilibrium constant expression.
- Solve for the unknown concentration(s).
- Check your answer.
Applications
Equilibrium reactions acids and bases have numerous applications in various fields, including:
- Buffer solutions: Buffers are solutions that resist changes in pH. They are used in a wide range of applications, such as maintaining a stable pH in biological systems.
- Titrations: Titrations are analytical techniques used to determine the concentration of an unknown solution. Acid-base titrations involve the reaction between an acid and a base to reach an equivalence point.
- Solubility: The solubility of ionic compounds in water is influenced by the equilibrium between the solid compound and its dissolved ions.
- Environmental chemistry: Acid-base reactions play a crucial role in understanding the chemistry of natural waters, soil, and the atmosphere.
Equilibrium reactions acids and bases are a fundamental concept in chemistry with applications in various fields. This quick review notes provide a comprehensive overview of the key concepts, equations, and problem-solving techniques essential for understanding this topic.
By mastering equilibrium reactions acids and bases, you can gain a deeper comprehension of chemical processes and enhance your problem-solving abilities. Whether you are a student, researcher, or professional, this guide will serve as a valuable resource for expanding your knowledge and expertise in this essential area of chemistry.
4.3 out of 5
| Language | : | English |
| File size | : | 105 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Print length | : | 6 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia D L Harrison
D L Harrison Dale Sattler
Dale Sattler Cullen Roche
Cullen Roche Walter R Paczkowski
Walter R Paczkowski Daily Language Learning
Daily Language Learning Rebecca Otowa
Rebecca Otowa Laura Shapiro
Laura Shapiro Jacklyn Williams
Jacklyn Williams Robert Stanek
Robert Stanek Criss Angel
Criss Angel Cliff Harris
Cliff Harris Mike Senior
Mike Senior Lamont Lindstrom
Lamont Lindstrom Smauggy Universe
Smauggy Universe Cp Lowe
Cp Lowe Stephen Clarke
Stephen Clarke Mildred D Taylor
Mildred D Taylor Crystal Allen
Crystal Allen Courtney Sheinmel
Courtney Sheinmel D S Allan
D S Allan
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!
 Ivan CoxFollow ·5.6k
Ivan CoxFollow ·5.6k Raymond ChandlerFollow ·14.4k
Raymond ChandlerFollow ·14.4k Jean BlairFollow ·12.5k
Jean BlairFollow ·12.5k Dale MitchellFollow ·14.2k
Dale MitchellFollow ·14.2k Floyd RichardsonFollow ·13.4k
Floyd RichardsonFollow ·13.4k Diego BlairFollow ·17.4k
Diego BlairFollow ·17.4k Jerome BlairFollow ·12k
Jerome BlairFollow ·12k Ethan MitchellFollow ·7.2k
Ethan MitchellFollow ·7.2k

 Cruz Simmons
Cruz SimmonsUnveiling the Secrets: An Insider Guide to School Bonds...
Unlock the Power of School...

 Gil Turner
Gil TurnerRuins of Empire: Blood on the Stars - The Epic Space...
Ruins of Empire: Blood on the Stars is the...

 Allen Ginsberg
Allen GinsbergPrepare for the Ultimate Space Opera: Delve into The Last...
Embark on an...

 Anton Foster
Anton FosterUnleash Your Inner Artist: The Ultimate Guide to Oil...
Chapter 1: The...
4.3 out of 5
| Language | : | English |
| File size | : | 105 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Print length | : | 6 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |